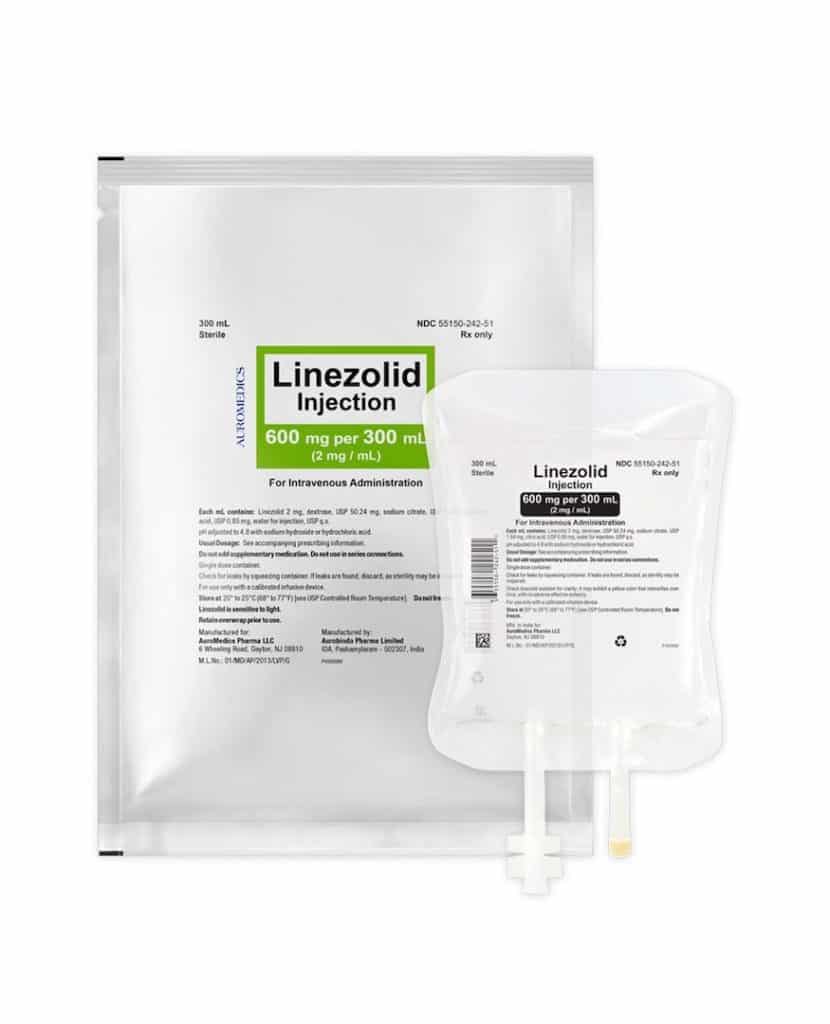
AuroMedics Pharma LLC has issued a voluntary recall of the company’s Linezolid Injection drug, the FDA reports. The hospital level recall includes 600mg/300mL flexible bags NDC 55150-242-51 batch CLZ160007 with an August 2018 expiration date. According to AuroMedics, the batch was distributed May 15 through August 14, 2017.
The pharmaceutical company recalled the drug after it was found to contain particulate matter, which has been identified as mold. The issue was discovered after white particulate matter was found in a bag from the recalled batch.
AuroMedics has not received any reports of identifiable safety concerns or adverse events related to the recall.
“Use of a non-sterile injectable product could result in fatal infections in a broad array of patients,” the FDA said in the report.
Linezolid is an oxazolidinone-class antibacterial used to treat pneumonia, certain skin infections and Vancomycin-resistant Enterococcus faecium infections.
The drug is supplied as a ready-to-use solution for intravenous infusion. Each 300 mL flexible bag contains 600 mg of linezolid. Inactive ingredients include citric acid, sodium citrate and dextrose. The sodium content of the injectable drug is 0.38 mg/mL (5 mEq/300 mL bag). The product is designed to be administered intravenously.
The single-use, ready-to-use bags are supplied in a foil laminate overwrap. Both the ports and infusion bags are latex-free.
The recalled batch was shipped to customers between May 15 and August 14, 2017. The product was distributed to hospitals and/or wholesalers across the nation.
AuroMedics will send recall letters to customers and distributers, and will be arranging for return or replacement of all recalled products. Patients are urged to talk to their doctors about any adverse events related to the drug.
Quality issues or adverse reactions related to this product can be reported to the FDA’s MedWatch Adverse Event Reporting program at https://www.fda.gov/MedWatch/report.htm.
Consumers who have questions about the recall can contact Aurobindo Customer Service at 866-850-2876 Option 1.