A number of personal injury lawsuits have recently been filed against the manufacturer of Elmiron,® alleging the drug causes serious vision problems that can lead to blindness, and the manufacturer failed to properly warn physicians and patients of this risk.
Elmiron used for bladder and urinary issues
Elmiron (pentosan polysulfate sodium) is the only oral medication approved for the treatment of interstitial cystitis, which causes chronic bladder pain, urinary frequency, and urinary urgency.
Over 1 million people in the United States are estimated to suffer from this condition. Elmiron is manufactured and distributed in the United States by Janssen Pharmaceuticals, Inc., upon license by Teva Pharmaceuticals.
How Elmiron works is not completely understood, although it is believed to block irritants in the urine from reaching the bladder wall by lining the bladder’s mucosal layer. This creates a protective coating of the bladder wall.
Approved in 1995, Elmiron still has sales of over $150 million annually and is one of the most commonly prescribed treatments for interstitial cystitis.
Elmiron Eye Damage
While Elmiron has been used to treat interstitial cystitis for a number of years, only recently has a link between Elmiron and maculopathy become public.
Maculopathy is a broad term that encompasses any condition affecting the macula. The macula is the rear part of the retina and is primarily responsible for our central vision and color vision, whereas the other portions of the retina are responsible for our peripheral vision.
In November 2018, the first of a number of case studies and journal articles linking Elmiron with maculopathy was released.
The Emory Eye Center published a case study of six patients who had developed pigmentary maculopathy without an apparent cause between May 2015 and October 2017. The common thread among these six patients was each had taken Elmiron long-term for the treatment of interstitial cystitis. All six patients underwent extensive imaging and testing to determine if the cause was genetic or pathogenic in origin, but no such causes were found.
In April 2019, physicians at the Emory Eye Center published an expanded case study of ten patients who developed pigmentary maculopathy after taking Elmiron for interstitial cystitis.
Appearing in the Journal of Urology, the case study provided details of the Elmiron dosage and duration for the ten patients with pigmentary maculopathy. The authors also noted there were 156 other patients treated at the Emory Eye Center who suffered from interstitial cystitis but did not use Elmiron, and none of these patients showed any signs of pigmentary maculopathy.
In October 2019, ophthalmologists at Kaiser Permanente released the findings of an examination of Kaiser’s entire database of 4.3 million patients for any cases of pigmentary maculopathy and Elmiron.
They identified 140 patients who took Elmiron long-term and persuaded 91 of these patients to come in for an eye examination. Twenty-two of the 91 patients were found to have clear signs of macular abnormalities due to Elmiron toxicity.
Researchers also noted the rate of toxicity increased with the amount of Elmiron consumed, with those patients taking 1,500 grams or more of Elmiron making up 42% of the cases of macular abnormalities, with those patients taking 500 to 1,000 grams comprised only 11%.
In October 2019, researchers at Harvard Medical School published a case study of a patient with Elmiron-related pigmentary maculopathy that continued to worsen six years after her Elmiron use ceased.
In November 2019, researchers at Emory Eye Center released the results of a matched cohort study of thousands of Elmiron users versus control patients, which revealed that after 7 years of use, the Elmiron users had a significantly increased risk of suffering from pigmentary maculopathy.
Elmiron Side Effects – Maculopathy
Because maculopathy is typically painless, many individuals do not immediately recognize some of the symptoms.
The most common maculopathy symptoms include:
- Image distortion or metamorphosis, where images appear distorted;
- Difficulty adjusting to dark lighting;
- An area or spot in the center of the field of vision where the individual cannot see;
- Difficulty focusing on objects.
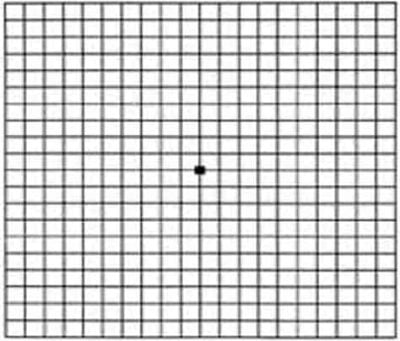
One of the most common methods of testing for potential maculopathy or the progression of maculopathy is the Amsler Test.
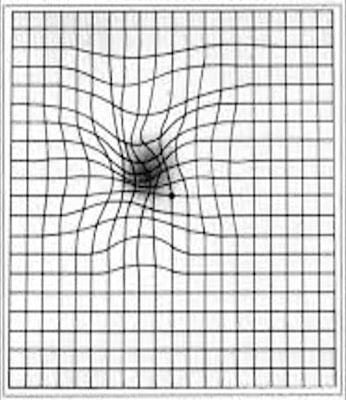
The Amsler test is designed to identify metamorphopsia, which is the most common symptom of maculopathy and is defined by the distortion or deformation of straight lines. If the patient sees distorted, curved, or broken lines, this is an indication of maculopathy.
Elmiron Lawsuits and Settlements
Over 700 Elmiron lawsuits were filed that allege Janssen Pharmaceuticals failed to warn doctors or patients that serious pigmentary maculopathy can develop from long-term Elmiron use.
The Elmiron label did not include any language describing pigmentary maculopathy or any type of maculopathy as a potential complication from Elmiron use.
Under pressure from the growing number of Elmiron lawsuits, on June 16, 2020, the FDA approved Janssen’s request to change the Elmiron warnings on the label to include the risk of retinal pigmentary “changes.”
Janssen now admits these permanent vision injuries can occur with both long and short-term use of the drug.
There is expected to be a resolution to the Elmiron lawsuits through a global settlement, either just before or after the bellwether trials. Based on this prediction, the timeline for the settlement will likely occur between April and June 2023, depending on whether the bellwether trial dates are moved.