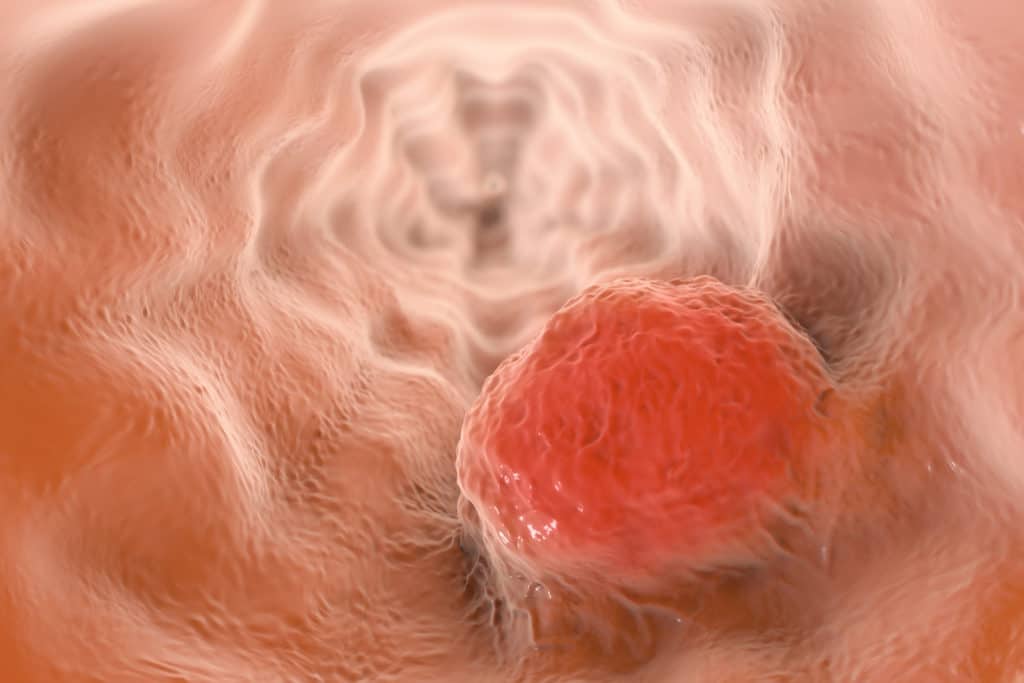
Last month, a study published in Cancer Epidemiology found a link between long-term proton pump inhibitor (PPI) use and esophageal cancer. The research used four Swedish registries to identify 796,492 patients with no history of cancer and exposure to proton pump inhibitor therapy between 2005 and 2014.
Most patients in the study were female, and approximately 34% were 70 years of age or older. The patients took proton pump inhibitors for various reasons, including peptic ulcer disease, gastroesophageal reflux disease, maintenance therapy with aspirin, and nonsteroidal anti-inflammatory drugs. The data was then compared to the general population of adults, matched for age and sex.
Researchers found that patients prescribed proton pump inhibitors, like Prevacid, Nexium, and Prilosec, for extended periods of time may develop esophageal cancer.
PPIs are used to protect the stomach lining and treat acid reflux. It is also used to treat the bacterial infection of H. pylori.
A previous study found that those treated for a stomach infection with H. pylori were more likely to be diagnosed with stomach cancer in the following 7-8 years after treatment.
Experts have been quick to warn that the risk of cancer is still low, and the results of the study don’t necessarily prove that PPI use is the root cause of the increased risk of cancer.
In 2013, 15 million Americans used PPIs, but it is estimated that between 25% and 70% of those prescriptions had no appropriate indication. Because they are so commonly prescribed, people rarely consider the potential side effects of long-term use.
Previous research has linked the long-term use of these medications to bone fractures, B12 deficiency, dementia, heart attacks, kidney issues, low magnesium levels, pneumonia, and C. diff infections.
Plaintiffs in cases targeted PPI manufacturers, having accused the drug makers of concealing information that links their products to serious kidney complications. They claim that their injuries could have been prevented had they been adequately warned of the risks and side effects.