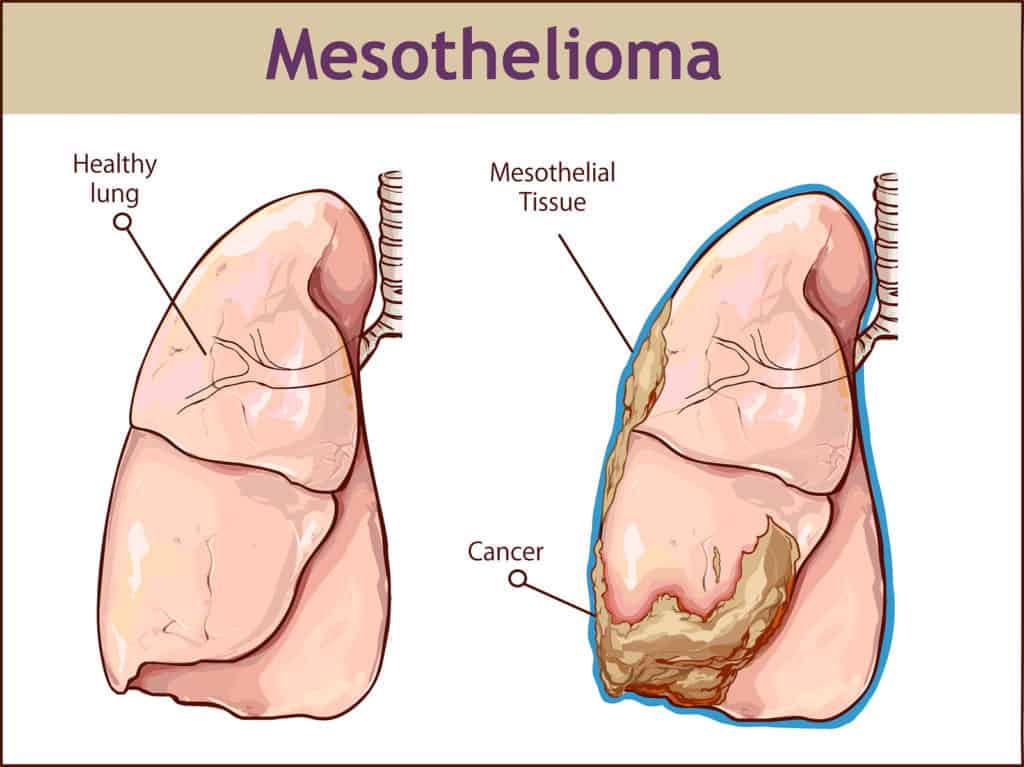
The Journal of Clinical Oncology released a phase 2 study which was conducted on 92 patients suffering from malignant pleural mesothelioma that have never had chemotherapy and all had tumors which could not be surgically removed. The patients, 85% being men, averaged 72 years in age and underwent a treatment that consisted of platinum-based chemotherapy and Cediranib.
The patients were part of a trial that took place between October 2011 and February 2016.
Patients were randomly selected to receive platinum-pemetrexed chemotherapy with either a placebo or Cediranib. Cediranib is still in the investigational phase and is known as a VEGFR. The oral medication is not approved by the FDA, so it’s not as widely available as other medications.
The medication works by preventing new blood vessels from growing, which is needed for tumors to grow. Cancer cells may also be killed due to this preventative step, but more research needs to be conducted to verify the impact Cediranib has on cancer cell death.
Researchers found that the combination of chemotherapy and Cediranbib was able to improve the progression-free survival rate from 5.6 months for those that were administered the placebo to 7.2 months.
Overall survival rates were not different, and it is to be noted that the patients that were given Cediranib suffered from more intense side effects, including weight loss, hypertension, dehydration, and grade 3 and 4 diarrhea.
Researchers note that the study was able to show that the drug increase response rates and was able to reduce tumor burden. The increase in response rate did not provide a significant increase in overall survival, so much more research needs to be done on the treatment to determine how it can translate into a positive impact on the fight against mesothelioma.
During the trial, two deaths occurred in the group that was administered Cediranib and two deaths in the placebo group.