C.R. Bard’s PowerPort™ series of vascular access devices or implantable ports are the subject of recent injury lawsuits and past settlements. The lawsuits allege the PowerPort is defectively designed and that Bard failed to adequately warn of risks associated with the implantation of the PowerPort.
The PowerPorts and port-a-caths are implanted under the patient’s skin and designed to deliver medication and other fluids into the patient’s bloodstream at a high flow rate. Vascular access devices like the PowerPort are most commonly implanted in cancer patients who will need frequent chemotherapy treatment over a long period.
Recent product liability lawsuits against Bard and its subsidiary, Bard Access System, Inc., allege that Bard’s PowerPort vascular access devices are susceptible to breaking, fracturing, or pinching off, increasing the risk of complications such as embolism, deep vein thrombosis (DVT), puncture of a vein, blood clots or infection.
As of late 2024, there are hundreds of product liability lawsuits against Bard for injuries allegedly caused by its PowerPort implantable port devices.
The injury lawsuits filed in federal court were recently consolidated before the Honorable David G. Campbell of the U.S. District Court for the District of Arizona.
Key Takeaways
- The Bard PowerPort lawsuits allege the vascular access device is defectively designed, which may lead to serious health risks.
- Individuals allege they were harmed by Bard’s PowerPort devices seek compensation for their injuries through individual lawsuits filed in federal (Arizona) and state courts (New Jersey).
- PowerPort refers to several vascular access devices marketed by Bard under the PowerPort trademark, not one specific device.
The PowerPort Port-a-Cath Lawsuits
Initially approved by the FDA in 2000, the Bard PowerPort is an implantable vascular access device that provides long-term, easy access to a patient’s vascular system so that medication and fluids can be delivered intravenously.
The Bard PowerPort lawsuits revolve around allegations that these vascular access devices manufactured by Bard have design flaws. These injury lawsuits allege that a design flaw in the catheter portion of the PowerPort can cause it to fissure, fracture, or crack and lead to a variety of serious complications for patients, particularly severe infections.
The catheter portion of the Bard PowerPort is comprised of a mixture of polyurethane and barium sulfate called Chronoflex AL. The PowerPort lawsuits allege that the amount of barium sulfate can adversely affect the mechanical integrity of polyurethane, especially in high concentrations.
According to the lawsuits, Bard’s manufacturing process uses too high a concentration of barium sulfate, causing structural changes and degradation of the catheter. Eventually, these structural changes and degradation can weaken the catheter’s integrity, causing it to fracture or “pinch-off,” leading to a variety of serious injuries.
Bard PowerPort patients have filed lawsuits against C.R. Bard and Bard Access Systems after experiencing complications such as the fracture or pinching-off of the catheter, migration of the catheter, and infection, which these patients allege were due to a defect in the design of the Bard PowerPort.
If the catheter breaks or fractures, fragments can enter the blood vessels, causing potentially life-threatening conditions like:
- pulmonary embolism
- vessel perforation
- blood clots
- cardiac tamponade
- infection
Likewise, catheter migration, which occurs when the catheter moves from its original position, can cause potentially life-threatening complications such as vessel perforation, pulmonary embolism, and blood clots.
Bard PowerPort patients who suffered these complications have filed lawsuits that seek compensation against C.R. Bard and Bard Access Systems for:
- medical bills
- pain and suffering
- lost income
- loss of future income
- loss of consortium
PowerPort Lawsuits in Federal and State Courts
As of November 2023, at least 70 individual injury lawsuits have been filed against C.R. Bard and Bard Access Systems on behalf of people who allegedly suffered injuries from the Bard PowerPort.
Product liability lawyers anticipated a significant number of additional lawsuits would be filed. Several experienced product liability law firms have taken on the cases against these defendants, seeking to obtain fair settlements (or verdicts) for their clients.
In July 2023, the U.S. Judicial Panel on Multidistrict Litigation consolidated all the federal Bard PowerPort lawsuits into MDL 3081 or In re Bard Implanted Port Catheter Products Liability Litigation and transferred all of these cases to the U.S. District Court for the District of Arizona. The cases were assigned to the Honorable David G. Campbell for pre-trial purposes.
Late-2024 Update
As of the most recent 2024 update, there are over 230 Bard PowerPort lawsuits consolidated in the federal Multidistrict Litigation (MDL) under Judge David G. Campbell in Arizona (MDL No. 3081). Additionally, there are efforts to consolidate over 500 lawsuits in New Jersey state courts through a multi-county litigation (MCL) process.
- Federal Court (MDL No. 3081): Over 720 cases.
- New Jersey State Court (MCL): Over 50 cases.
These numbers indicate significant legal activity both at the federal and state levels, reflecting the widespread nature of the claims against Bard PowerPort devices.
AngioDynamics Port Catheter Lawsuits
Cases Consolidated in San Diego, California
In a further development, the U.S. Judicial Panel on Multidistrict Litigation has centralized all AngioDynamics port catheter lawsuits in the Southern District of California, specifically before U.S. District Judge Jinsook Ohta.
This consolidation, established on October 7, 2024, brings together over 50 product liability claims against AngioDynamics, mirroring the approach taken with the Bard PowerPort litigation.
Key details of the AngioDynamics MDL include:
- The MDL is officially designated as MDL No. 3125.
- At the time of consolidation, there were at least 23 lawsuits pending in 16 different district courts, with 33 additional potential actions identified in 19 districts.
The lawsuits allege that AngioDynamics’ port catheter devices are defective, leading to serious health risks such as:
- Device fractures
- Catheter migration
- Severe infections
- Blood clots
- Organ damage
Plaintiffs claim that these complications arise from the catheter’s manufacturing process, specifically the use of excessive barium sulfate. This excess allegedly causes the material to degrade, leading to pitting or cracking of the catheter surface.
As of November 2024, the number of cases in the AngioDynamics MDL has grown to 62, with 12 new cases added in the past month alone.
The consolidation of these cases in San Diego, California is expected to streamline the legal process. As the cases progress, it will be closely watched alongside the Bard PowerPort lawsuits, given the similarities in allegations and the medical devices involved.
Implantable Port and Port a Cath Complications
Adverse Event Reports and Safety Concerns
Due to the risks medical devices may pose to patients, as well as the inability of the U.S. Food and Drug Administration to effectively evaluate medical devices for safety and efficacy, any post-release adverse events associated with a medical device must be appropriately documented and publicly reported.
This is the only means by which the FDA, physicians, and the public can identify potential defects or safety issues with a medical device. For years, adverse events reported to the FDA’s publicly accessible Manufacturer and User Facility Device Experience database (“MAUDE”) were how surveillance for potential defects and safety issues was done.
However, there are significant deficiencies in this system. Surveillance of the MAUDE database for safety issues is only effective if adverse events associated with a medical device are reported to the database accurately and timely.
Unfortunately, the reporting of adverse events to the MAUDE database is typically done by the medical device manufacturer or physicians, who are often too busy to report most adverse events to either the medical device manufacturer or MAUDE.
Compounding the inadequacies of using the MAUDE database to surveil for potential defects and safety issues with medical devices was the recent discovery that many adverse events associated with medical devices are not even placed into the publicly accessible MAUDE database.
In 2019, Kaiser Health News (now KFF) discovered that the FDA had allowed medical device manufacturers to hide adverse health events from the public via the use of the FDA’s Alternative Summary Reporting program, which permits certain medical device manufacturers, including C.R. Bard, to file adverse event reports into a non-public, internal FDA database instead of the public MAUDE database.
Established by the FDA in 2002, this program has allowed medical device manufacturers to hide millions of adverse event reports from the public. This program also allowed these manufacturers to file “summary reports” instead of individual reports for each adverse event required by the MAUDE database.
Due to public pressure, the FDA ended this secret AER database and released more than 5.7 million adverse event reports not previously known to the public.
A review of the secret AER database by Kaiser Health found that certain medical devices comprised a significant number of the adverse events in this database, including surgical staplers, balloon pumps for blood vessels, and vascular access devices, including Bard’s PowerPort.
Bard was able to hide adverse events due to “compression or pinch-off events” in its PowerPort devices by claiming these complications were “known risks” associated with these devices or were the result of an error on the part of the surgeon in implanting these devices.
This allowed Bard to circumvent reporting these adverse events into the public MAUDE database. The discovery of the 5.7 million adverse event reports hidden from public view by the FDA and certain medical manufacturers, along with the significant number of these events associated with Bard’s PowerPort devices, helped prompt these lawsuits.
Since medical devices like the Bard PowerPort are often critical components in a patient’s treatment plan, healthcare professionals and patients need to know about potential risks and recall information.
Compared to medications and therapy, the stakes are even higher, as improperly functioning medical devices can hinder treatment efficacy, introduce new health risks, and, in severe cases, result in serious injury or death.
Being fully informed about potential safety issues associated with devices like the Bard PowerPort can empower patients and medical professionals to make informed decisions regarding their treatment options.
By staying current on safety issues and advocating for patient well-being, the community can help minimize adverse outcomes and ensure that quality care remains a priority.
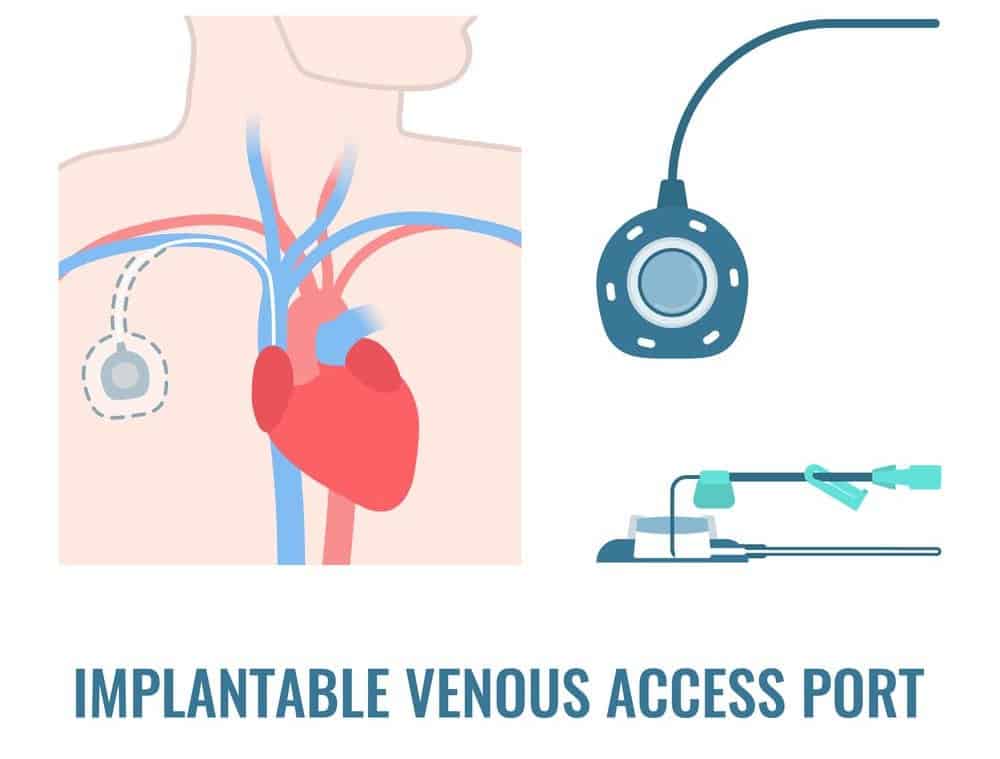
Implantable PowerPort Device and Design
When treating cancer, a crucial aspect is the delivery of chemotherapy drugs. Implantable ports and catheter tubes play a significant role in ensuring patients receive medication without constant needle insertions.
A popular device for this purpose is the Bard PowerPort series of vascular access devices manufactured by C.R. Bard and its subsidiary, Bard Access Systems.
The Bard PowerPort Devices are made with various materials but are typically comprised of polyurethane or silicone for the device’s body and a mixture of polyurethane and barium sulfate for the catheter tube.
The barium sulfate is used to allow the catheter to be visible in imaging procedures so that physicians can ensure proper placement of the catheter.
However, as the Bard PowerPort lawsuits allege, the use of this barium sulfate, or at least high concentrations of it, can degrade and alter the catheter’s structural integrity, leading to fracture, breaking, or fragmentation.
These lawsuits further allege that the catheter in the Bard PowerPort devices can also migrate from its original position. The fracture, fragmenting, or migration of the catheter can also lead to a series of severe and potentially life-threatening complications.
Seeking Compensation for Injuries
If you experienced any of the following conditions post-implantation of a PowerPort device, you might be eligible for a lawsuit against the manufacturer:
- Infections: You may qualify if you suffered sepsis, septic shock, or severe infections that can lead to organ damage or death.
- Deep Vein Thrombosis (DVT): A DVT is a blood clot in a deep vein, typically in the leg. It can be life-threatening if it travels to the lungs, causing a pulmonary embolism.
- Hemorrhaging or Bleeding Injuries: Uncontrollable bleeding is a serious condition requiring immediate medical attention.
- Fluid Buildup on the Heart: Known as pericardial effusion, this can cause shortness of breath, chest pain, and other heart-related problems.
- Irregular Heartbeat: An abnormal heart rhythm known as arrhythmia can lead to serious health complications, including stroke and heart failure.
- Severe and Persistent Pain: Chronic pain, particularly in the chest or neck, could indicate a device malfunction.
- Perforations of Tissues, Vessels, and Organs: If the PowerPort catheter has punctured any tissues, vessels, or organs, this is a severe complication.
- Wrongful Death: If a loved one passed away due to any of the above complications linked to the Bard PowerPort™ device, you may be able to file a lawsuit on their behalf.
- Other Injuries: If you experienced injuries due to a fractured PowerPort catheter, you may still be eligible to file a claim.
The journey toward justice can be daunting, but you don’t have to navigate it alone. If you’ve faced any of these complications following the implantation of a Bard PowerPort device, please speak to an experienced products liability attorney about your potential legal options.
You may be entitled to seek significant financial compensation through a Bard PowerPort lawsuit, providing some recompense for the physical, emotional, and economic toll these complications have caused.
Implantable Port-a-Cath Lawsuit FAQ
What are some common injuries and complications linked to the implantable PowerPort?
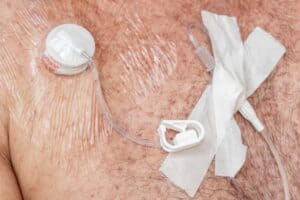
Bard PowerPort vascular access devices are implanted devices designed to access patients’ veins easily. However, the following adverse events have been reported with Bard’s PowerPort devices:
- Break
- Fracture
- Embolism
- Infection
- Vein and organ embedding
- Vein and organ perforation
- Thrombosis
How have patients been allegedly harmed by the implantable PowerPort device?
Patients affected by Bard PowerPort devices allegedly suffer from infections, injuries, or other health issues related to defects in the product. These complications can result in additional medical treatments, hospitalizations, severe health consequences, or even death in extreme cases.
What is claimed in the PowerPort implant lawsuits?
The Bard PowerPort lawsuits primarily focus on product liability. Bard, the manufacturer of these medical devices, is being sued for injuries and complications allegedly caused by defects in their PowerPort products and for failing to adequately warn physicians and patients of the severity and frequency of complications associated with the Bard PowerPort devices.
Has Bard settled any implantable PowerPort lawsuits?
To date, no settlements have been reached in any of the recently filed Bard PowerPort lawsuits. This is not surprising, given that the lawsuits are still in their early stages.
What are the potential outcomes for plaintiffs in these power port lawsuits?
While the outcomes of Bard PowerPort lawsuits can vary, plaintiffs who successfully prove that Bard PowerPort caused their injuries could receive compensation, including medical expenses, lost wages, and pain and suffering.
In some cases, plaintiffs could also be awarded punitive damages if it’s determined that Bard acted recklessly or acted with reckless disregard for patient safety.
How can someone injured by PowerPort join or start a lawsuit?
Those who feel they may have suffered complications from one of Bard’s PowerPort devices should consult a knowledgeable medical device attorney by filling out the form below.
An experienced attorney can evaluate their specific situation and determine if they may have a viable legal claim. It’s essential to act promptly, as there are time limits for legal action to be taken.
Fill out the contact form for a free case and medical record review.
Start a free, no-obligation claim review today
with an experienced medical device lawyer.
- Pay nothing unless you win
- Millions recovered
- 26+ years experience



